Drug Repurposing
Drug repurposing refers to the research method of reusing previously approved drugs for treating different diseases. It involves developing drugs, which were initially halted at the development stage, for alternative therapeutic purposes. This approach offers the advantage of developing new drugs more quickly and efficiently.
This method is gaining greater value with the increase of the aging population. Moreover, the combination of AI with pharmaceutical companies can further expand this value. In the past, drug repurposing was reliant on experience or chance, but now, it can gain efficiency through AI, making it a promising area.
This topic is continuously emphasized in publications like ‘Global Trends 2040’, ‘The Power of Reading 1% of Merck’, and ‘2040 Future Predictions’. It’s an area that warrants continuous attention for future investment success.
Examples of drug repurposing
Drug Repurposing Case #1: Disulfiram
In 1971, a 38-year-old cancer patient became addicted to alcohol. The patient had widespread metastases and gave up on treatment. To at least address the alcohol addiction, the patient took Disulfiram for 10 years. However, it didn’t treat the alcohol addiction. One day, heavily intoxicated, the patient passed away. Upon autopsy, it was discovered that the pre-existing tumors had disappeared. This discovery suggested a potential anticancer effect of Disulfiram, a drug that wasn’t very effective for alcohol addiction. Clinical trials were conducted based on this finding, and the survival rate for cancer patients taking Disulfiram was found to be 76%.”
Drug Repurposing Case #2: Viagra
Originally, Viagra was developed as a treatment for angina. Research has emerged suggesting that when administered alongside a flu vaccine, it can reduce cancer cell metastasis.
Drug Repurposing Case #3: Minoxidil
Minoxidil was originally tested as a medication to expand blood vessels and treat high blood pressure. While expanding blood vessels can reduce blood pressure, it also facilitates improved blood flow to hair follicles, which can promote hair growth. Consequently, it has been applied as a treatment for hair loss.
Drug Repurposing Case #4: Remdesivir
Remdesivir was initially developed by the U.S. pharmaceutical company Gilead Sciences as a treatment for the Ebola virus. However, it failed in Phase 3 clinical trials. Amidst the lack of treatments for COVID-19, animal studies confirmed that Remdesivir can inhibit the proliferation of the virus, leading to its rise in attention as a potential treatment for COVID-19.”
Market Outlook for Drug Repurposing
The Compound Annual Growth Rate (CAGR) of the global drug repurposing market shows some variation in forecasts by different sources, but it gives an overall idea of the expected market growth by 2030:
Growth Market Reports: This source projects that the global drug repurposing market, which was valued at USD 31.3 billion in 2020, will reach USD 46.85 billion by 2028, growing at a CAGR of 5.4%.
Industry Growth Insights: According to this report, the global drug repurposing market is expected to grow at a much higher CAGR of 10.8% during the period from 2018 to 2030. The growth is attributed to factors such as the increasing prevalence of chronic diseases, rising demand for novel therapies, and a growing focus on personalized medicine.
Stratistics MRC via GIIR Research: This analysis estimates the Global Drug Repurposing Market at $38.7 billion in 2023, forecasting it to reach $63.9 billion by 2030. This suggests a growth at a CAGR of 7.4% during the forecast period.
Market.us: This source provides a slightly lower growth projection, stating that the global drug repurposing market is anticipated to grow from US$ 27.34 billion in 2021 to US$ 39.14 billion by 2031, at a CAGR of 3.8%. These varying forecasts reflect different methodologies and market considerations. However, they collectively indicate a positive growth trend in the drug repurposing market, albeit at differing rates. The higher CAGRs suggest more optimistic views of market dynamics and potential, while the lower ones might be more conservative estimates.
Companies of Drug Repurposing
Algernon Pharmaceuticals
Algernon Pharmaceuticals Inc. operates as a clinical stage pharmaceutical development company. It focuses on the areas of nonalcoholic steatohepatitis, chronic kidney disease, inflammatory bowel disease, idiopathic pulmonary fibrosis, chronic cough, pancreatic and small cell lung cancer, and acute lung injury in Canada, Australia, and New Zealand. The company’s pipeline includes Ifenprodil, which completed Phase 2 clinical trial for the treatment of idiopathic pulmonary fibrosis and chronic cough; repirinast, which is in preclinical stage for the treatment of chronic kidney disease; and N,N-Dimethyltryptamine, which has completed preclinical stage for the treatment of strokes. The company was formerly known as Breathtec Biomedical, Inc. and changed its name to Algernon Pharmaceuticals Inc. in July 2015. Algernon Pharmaceuticals Inc. was incorporated in 2015 and is headquartered in Vancouver, Canada.
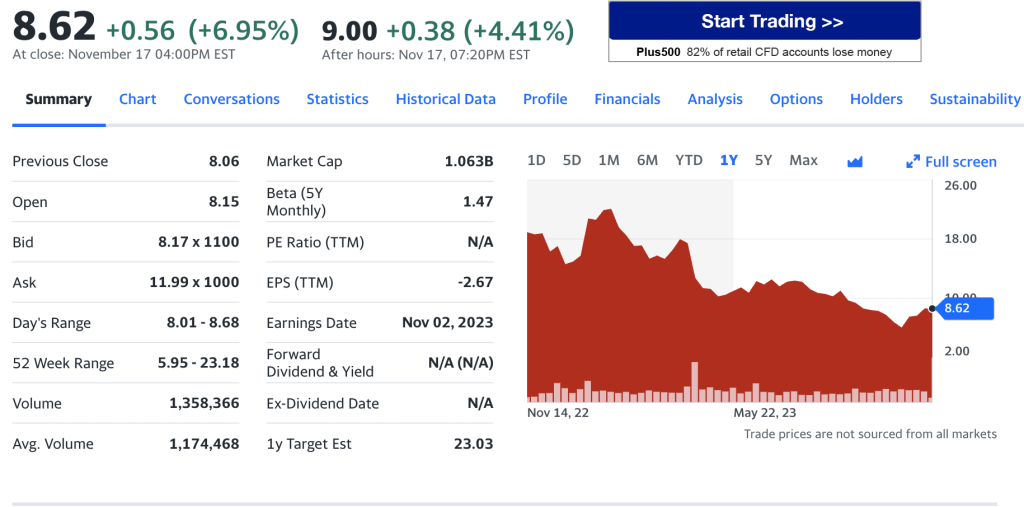
AgeX Therapeutics, Inc. (AGE)
AgeX Therapeutics, Inc., a biotechnology company, focuses on the development and commercialization of novel therapeutics targeting human aging and degenerative diseases in the United States. The company’s lead cell-based therapeutic candidates in development include AGEX-BAT1, a cell therapy product candidate for the treatment of various age-related metabolic disorders, such as Type II adult-onset diabetes and obesity; and AGEX-VASC1, a cell-based therapy to restore vascular support in aged ischemic tissues, such as peripheral vascular disease and ischemic heart disease. Its lead small molecule drug-based therapeutic candidate for scarless wound repair in discovery is AGEX-iTR1547, a drug-based formulation; and lead biologic candidate for induced tissue regeneration is AGEX-iTR1550 (Renelon), a gene delivery technology. AgeX Therapeutics, Inc. has a research collaboration with the University of California at Irvine to develop cellular therapies to treat neurological disorders and diseases. The company was incorporated in 2017 and is based in Alameda, California.
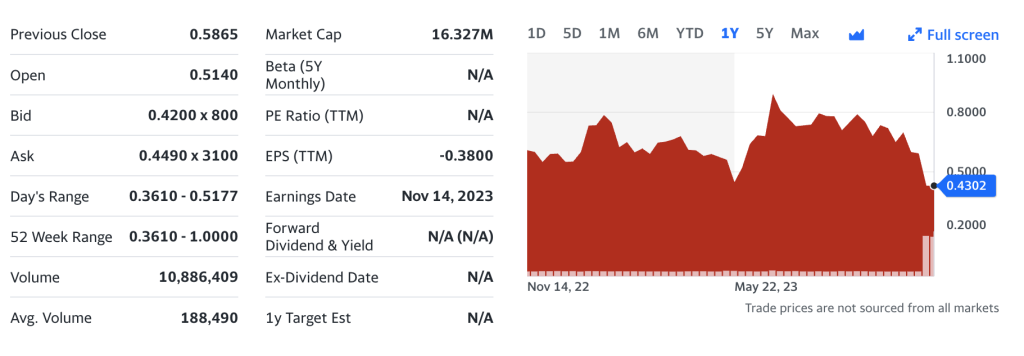
Recursion Pharmaceuticals.
Recursion Pharmaceuticals, Inc. operates as a clinical-stage biotechnology company, engages in the decoding biology by integrating technological innovations across biology, chemistry, automation, data science, and engineering to industrialize drug discovery. The company develops REC-994, which is in Phase 2 clinical trial to treat cerebral cavernous malformation; REC-2282, which is in Phase 2/3 clinical trial for the treatment of neurofibromatosis type 2; REC-4881, which is in Phase 2 clinical trial to treat familial adenomatous polyposis; REC-3964, which is in Phase 1 clinical trial to treat Clostridioides difficile infection; and REC-4881, which is in Phase 1b/2 clinical trial to treat AXIN1 or APC mutant cancers. Its preclinical stage product includes RBM39 to treat HR-proficient ovarian cancer; REC-64151 for the treatment of immune checkpoint resistance; and Anti-PD-(L)1, an orally bioavailable small molecule to improve sensitivity to immune checkpoint inhibitors in non-small cell lung cancer and additional tumors. The company has collaboration and agreement with Bayer AG; the University of Utah Research Foundation; Ohio State Innovation Foundation; Chromaderm, Inc.; Roche & Genentech; and Takeda Pharmaceutical Company Limited. Recursion Pharmaceuticals, Inc. was incorporated in 2013 and is headquartered in Salt Lake City, Utah.
Relay Therapeutics, Inc. (RLAY)
Relay Therapeutics, Inc. operates as a clinical-stage precision medicines company. It engages in transforming the drug discovery process with an initial focus on enhancing small molecule therapeutic discovery in targeted oncology and genetic disease indications. The company’s lead product candidates include RLY-4008, an oral small molecule inhibitor of fibroblast growth factor receptor 2 (FGFR2), which is in a first-in-human clinical trial for patients with advanced or metastatic FGFR2-altered solid tumors; RLY-2608, a lead mutant-PI3Ka inhibitor program that targets phosphoinostide 3 kinase alpha; and GDC-1971, an oral small molecule inhibitor of protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-2 that is in Phase 1 trial in patients with advanced solid tumors. In addition, it has collaboration and license agreements with D. E. Shaw Research, LLC to research certain biological targets through the use of D. E. Shaw Research computational modeling capabilities focused on analysis of protein motion to develop and commercialize compounds and products directed to such targets; and Genentech, Inc. for the development and commercialization of GDC-1971. The company was formerly known as Allostery, Inc. and changed its name to Relay Therapeutics, Inc. in December 2015. Relay Therapeutics, Inc. was incorporated in 2015 and is headquartered in Cambridge, Massachusetts.